What Mean Bond Length
1 on a question What do you mean by bond length. Measurement of bond length or distance is an average.

Bond Length And Bond Strength Chemistry Steps
Bonds and bond portfolios will rise or fall in value as interest rates change.

What mean bond length. Bonds vary between atoms depending on the molecule that contains them. The Strength of Sigma and Pi Bonds. Bond length measures the distance between the two nuclei in a covalent bond.
The reverse would be true about the bond lengths. As a general trend bond length decreases across a row in the periodic table and increases down a group. In the case of a covalent bond the contribution from each atom is called covalent radius of that atom.
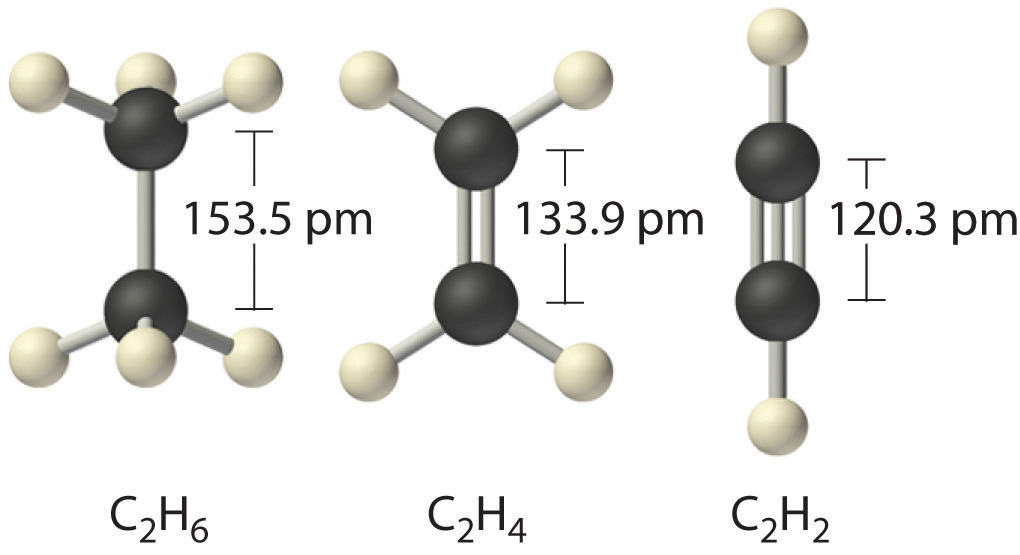
14012017 Bond length is the distance between two nuclei of atoms that are covalently bonded together. Going back to our tennis ball and rubber band example we. Bond length is the measurable distance between atoms covalently bonded together.
The important thing about bond length is its relationship with bond energy. Remember that a multiple bond consists of one σ and one or two π bonds. In a covalent bond the two atoms are held together because both nuclei are attracted to the same pair of electrons.
Bond length is the experimentally determined average distance between two bonded atoms. 15082020 Bond length is defined as the distance between the centers of two covalently bonded atoms. Bond lengths are typically in the range of 100-200 pm 1-2.
Each atom of the bonded pair contributes to the bond length. The bond length refers to the distance between the centers of the nuclei of two bonded atoms in an equilibrium position. When two similar atoms are bonded together half of the bond length is referred to as covalent radius.
16052012 The bond length of the covalent bond is the nuclear seperation distance where the molecule is most stableOr in simple words bond length is the distance between the nuclei in a bondThe H-H bond length in moleular hydrogen is 74 pmAt this distanceattractive interactions are maximied relative to repulsive interreactions. Bond length is defined as the distance between the centers of two covalently bonded atoms. Correlations between the observed mean bond lengths and the mean electronegativity of the cations in the structure R2 001030 and between the effective ionic radius the mean bond length minus the radius of O2 as a function of coordi-nation number and the mean electronegativity of the cations in the structure R2 046072.
The sensitivity to changes in the interest rate environment is called duration The use of the term duration in. Bond length is a measure of the distance between the nuclei of two chemically bonded atoms in a molecule. Bond length is usually in the range of 01 to 02 nm.
Bond length depends on the number of bonded electrons of two atoms or the bond order. It is a transferable property of a bond between atoms of fixed types relatively independent of the rest of the molecule. 13012020 In chemistry bond length is the equilibrium distance between the nuclei of two groups or atoms that are bonded to each other.
Bond length is defined as the equilibrium internuclear separation distance of the bonded atoms in a molecule. There is one important thing we should address when comparing the strength of a single bond with a double or a triple bond. Bonded atoms vibrate due to thermal energy available in the surroundings.
The higher the bond order the stronger the pull between. The higher the bond order the stronger the pull. The relationship between cell volume mean bond length and effective ionic radius.
The length of the bond is determined by the number of bonded electrons the bond order. Now if we compare the single bond strength with the double bond we have 88 kcalmol 152 kcalmol. The length of the bond is determined by the number of bonded electrons the bond order.
The stronger the force of attraction in between the bonding atoms the smaller is the length of the bond. Bond length In molecular geometry bond length or bond distance is the average distance between nuclei of two bonded atoms in a molecule. Bond length is a property of a chemical bond between types of atoms.
It is approximately equal to the sum of the covalent radii of the two bonded atoms. However the bigger the atom size the longer the bond length.

Bond Lengths Introduction To Chemistry
Https Labs Chem Ucsb Edu Zakarian Armen 01 03 Lecture 01 22 2018ppt Pdf
Bond Order Lengths And Strengths

Learn Quiz On Bond Energy Bond Length A Level Chemistry Quiz 26 To Practice Free Chemistry Mcqs Questions And An Bond Length Chemistry This Or That Questions
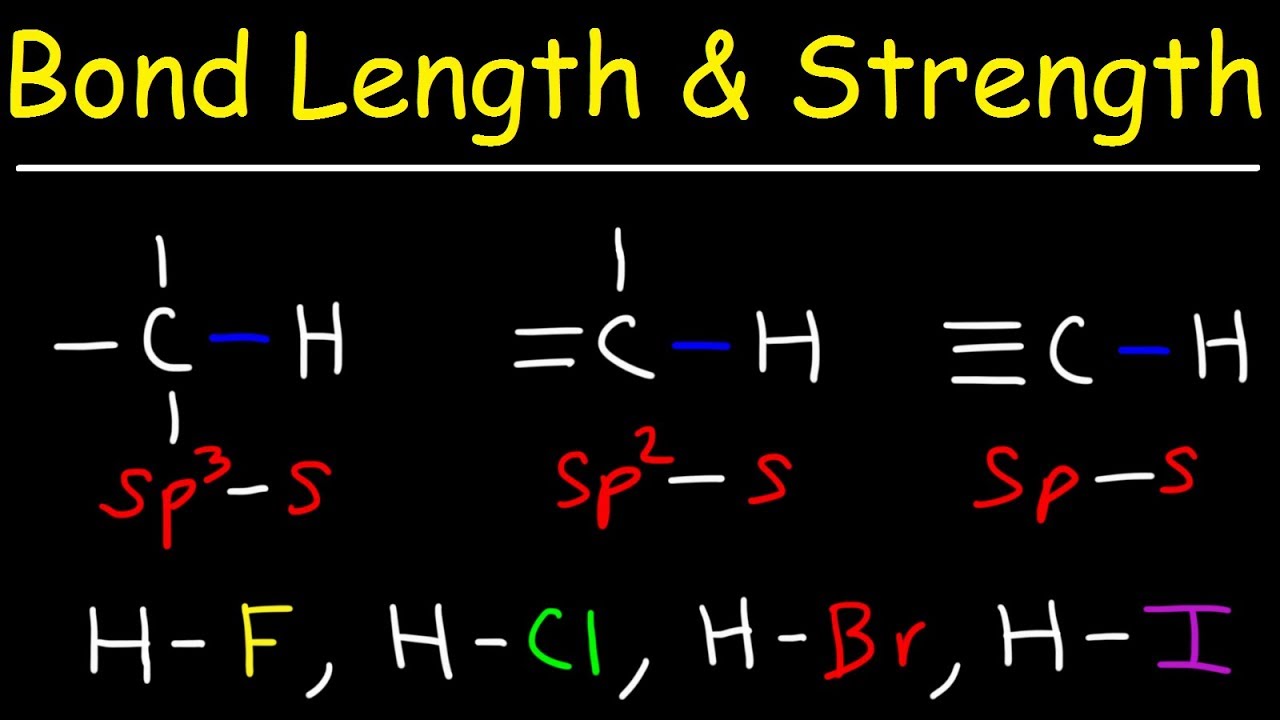
Bond Strength And Bond Length Youtube

Foundation Words Inspirational Quotes Love Quotes

Bond Length And Bond Strength Chemistry Steps