Non Covalent Bond Meaning
Ionic bond are non directional because it the electrostatic force between two opposite charges Hence bonding direction does not matter. A Nonpolar is termed as covalent bond because the difference in electronegativity is mostly negligible.

Covalent Bond Definition Types And Examples
27092020 Difference Between Covalent and Noncovalent Bonds.

Non covalent bond meaning. For many molecules the sharing of electrons allows each atom to attain the equivalent of a full valence shell corresponding to a stable electronic configuration. September 27 2020 Posted by Madhu. Farlex Partner Medical Dictionary.
Which bond is the longest. Non-polar Covalent Bonds When electrons are shared equally between two atoms then a type of nonpolar covalent bond is formed. Whereas covalent is directional as attraction is in a specific direction and at an angle relative to the bonding atoms.
In organic chemistry covalent bonds. These shared electrons glue two or more atoms together to form a molecule. 15032021 Definition of covalent bond.
These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. The monomers are chosen to allow hydrogen bonds ionic interactions π π interactions andor hydrophobic interactions with the print molecules. Inter-molecular forces of non-covalent bond between antigen and antibody is divided into four basic types.
The bond length of carbon-carbon C-C in diamonds is 154 pm. Non-covalent molecular imprinting relies on multiple non-covalent interactions between the print molecules and the monomers. In the case of covalent.
In an atom the number of electrons shared by the adjacent atoms will be the same. The force between oppositely charged ionic groups on the two protein side chains is known as electrostatic forces Fig. Note that this must occur between two nonmetal atoms in order for it to be a proper nonpolar covalent bond.
Of the intramolecular bonds or chemical bonds is the ionic bond then polar covalent bond and the strongest is the non-polar covalent bond. Not of the nature of or involving a covalent bond. Bond in which electrons are not shared between atoms for example electrostatic bond hydrogen bond.
Since the two atoms share the same electronegativity the bonds in molecular oxygen are nonpolar covalent. In molecules with a covalent non-polar bond common electron pairs are located at equal distances from the atomic nuclei. 16042019 Covalent bonding occurs between two non-metallic atoms characterized by the sharing of electron pairs between the atoms and other covalent bonds with electronegativity difference is greater than 20 20.
04012021 Nonpolar covalent bonds. Nonkō-vālĕnt bond Bond in which electrons are not. It is a bond that is formed between two identical atoms.
Molecular oxygen O 2 is made from an association between two atoms of oxygen. This bond is non-directional meaning that the pull of the electrons does not favor one atom over another. A chemical bond formed between atoms by the sharing of electrons.
28052019 Nonpolar covalent bonds are bonds where both atoms possess the same electronegativity and therefore the electrons in the electron bond are shared equally between them. Nonpolar Covalent Bond A nonpolar covalent bond definition is simple. Another example of a nonpolar covalent bond is the C-H bond found in the methane gas CH 4.
05082020 HH Nonpolar Covalent Bonds An example of a nonpolar covalent bond is the bond between two hydrogen atoms because they uniformly share the electrons. Unlike the case of molecular oxygen where the two bonded. 11032013 Nonpolar covalent bonds are a type of bond that occurs when two atoms share a pair of electrons with each other.
The key difference between covalent and noncovalent bonds is that covalent bonds form when two atoms share their electrons with each other whereas noncovalent bonds form either by completely exchanging electrons between two atoms or by not exchanging any electron. Nonpolar covalent bond A covalent bond in which the pair of electrons is shared equally between two atoms. 23062021 To understand the nature of noncovalent interactions we first must return to covalent bonds and delve into the subject of dipoles.
Many of the covalent bonds that we have seen between two carbons for example or between a carbon and a hydrogen involve the approximately equal sharing of electrons between the two atoms in the bond. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they. A nonpolar covalent bond picture.
02092001 A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.

Ib Chemistry Standard Level Notes Covalent Bonding

Noncovalent Bond The Bumbling Biochemist

Covalent Bonding Chemical Bonding Covalent Bonding Chemical Bond

Nonpolar Covalent Bond Definition And Examples

Ionic Bond Definition Examples Formation Digital Kemistry Ionic Bonding Ionic Chemical Bond
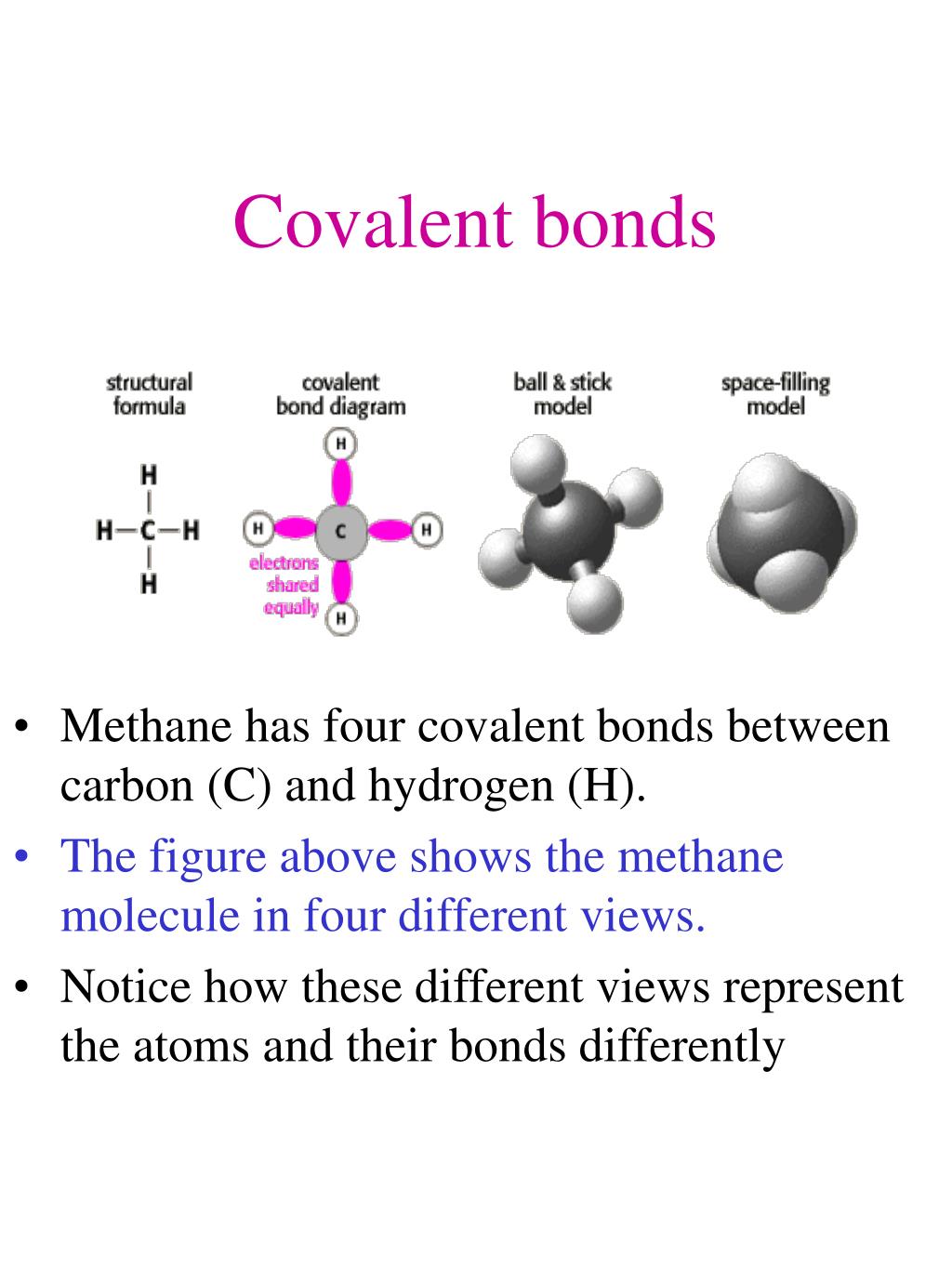
Ppt Covalent Bonds Powerpoint Presentation Free Download Id 6658311

Covalent Bond Animation Chemistry Covalent Bonding Chemistry Education Chemistry Animation
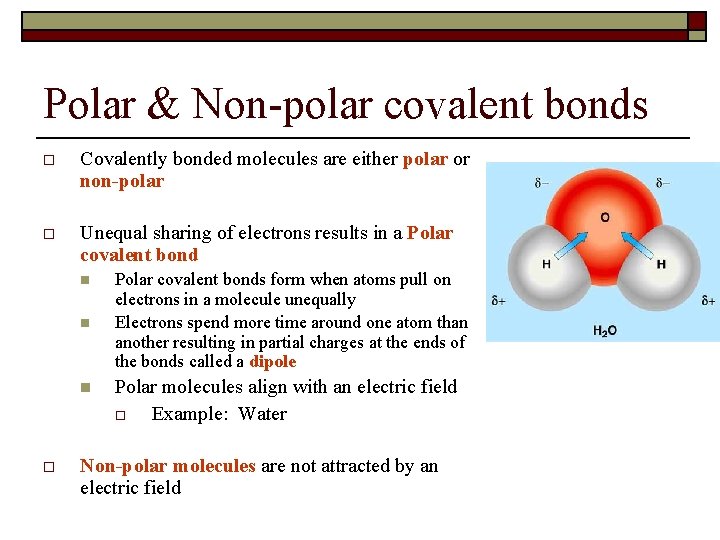
Covalent Bonding Chemistry Chapter 8 Objectives O O
