What Is The Meaning Of Metallic Bond
Metallic bonds are the chemical bonds that join metals to metals. 15082020 Metallic bonds occur among metal atoms.
Metallic Bonds And Properties Of Metals Metals Metals
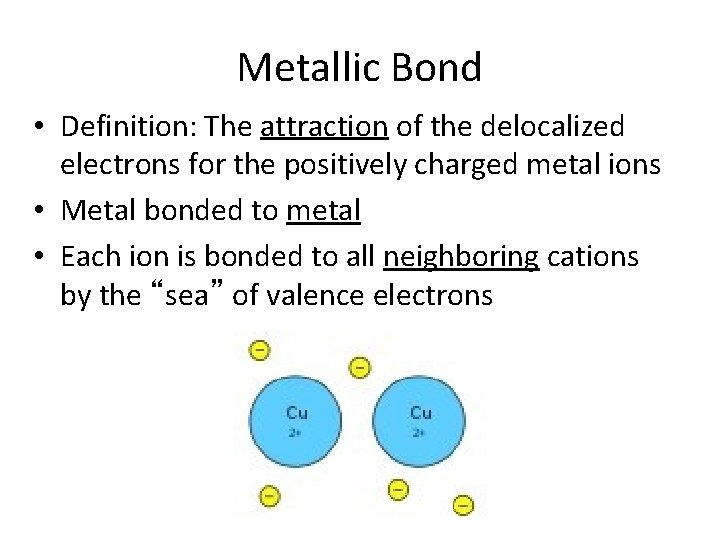
It may be described as the sharing of free electrons among a structure of positively charged ions cations.

What is the meaning of metallic bond. Ionic bonds join metals to non-metals. If a small stress is put onto the metal the layers of atoms will start to roll over each other. The ionic bond is the electrostatic force of attraction between two oppositely charged ions.
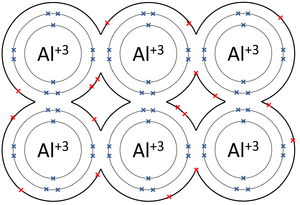
The attraction between the metals ions and the free floating electrons in the lattice structure of metal results in the formation of metallic bond. This bond is formed from the attraction between mobile electrons and. The metallic bond is a type of chemical bond that occurs between atoms of metallic elements.
It gives metals their unique properties that we dont see in nonmetal substances as youll learn in. The metallic bond is the force of attraction between these free-moving delocalised electrons and positive metal ions. Metallic bonding is a type of chemical bonding and is responsible for several characteristic properties of metals such as their shiny lustre their malleability and their conductivities for heat and electricity.
Metallic bonds are strong so metals can maintain a regular structure and. Such a solid consists of closely packed atoms. Metallic bonds are formed from the attraction between mobile electrons and fixed positively charged metallic atoms.
How does metallic bonds formed. Mə-tălĭk The chemical bonding that holds the atoms of a metal together. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action.
15082020 Metals are described as malleable can be beaten into sheets and ductile can be pulled out into wires. A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together Click again to see term 132 THIS SET IS OFTEN IN FOLDERS WITH. The type of bond occurring in metals in which the valence electrons are not localized as in covalent bonds but are capable of interacting with an indefinite number of the metal nuclei which are arranged in a lattice formation.
This is because of the ability of the atoms to roll over each other into new positions without breaking the metallic bond. Metallic bond is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions. The chemical bond typical of the metallic state and characterized by mobile valence electrons that hold the atoms together usually in crystal lattices and are responsible for the good electrical and heat conductivity of metals.
07092019 A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. The chemical bonding that holds the atoms of a metal together. When we think of the type of bondings in metals we should take into consideration the difficulty in separating metallic atoms from each other metals are hard materials with the possibility of moving them around malleability and ductility.
Moreover the conductivity of electricity and heat in all directions. Whereas ionic bonds join metals to non-metals metallic bonding joins a bulk of metal atoms. Definition of metallic bond.
Whereas most chemical bonds are localized between specific neighboring atoms metallic bonds extend over the entire molecular structure. 23112017 Metallic Bond Definition Metallic bonding is a special type of bonding that holds the metals together in metal crystal. This bond is neither covalent nor ionic.
Metallic bonds result from the sharing of a variable number of electrons by a variable number o. In contrast covalent and ionic bonds form between two discrete atoms. The principal force holding together the atoms of a metal.
The metallic bond is a type of chemical bond that occurs between atoms of metallic elements. Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons in the form of an electron cloud of delocalized electrons and positively charged metal ions. What are Metallic bonds.
In most cases the outermost electron shell of each of the metal atoms overlaps with a large number of neighbouring atoms. Definition of Metallic Bond. The covalent bond is also called a shared bond.
Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. 07102020 In simple words metallic bond is the type of Chemical bond formed between the metal atoms. Metallic bond force that holds atoms together in a metallic substance.
Metals have tendency to give up electrons and none is their to accept it. These bonds join non-metals to non-metals. If the stress is released again they will fall back to their.
Taking all these facts into consideration the bonds.

Metallic Bonding And The Electron Sea Model Electrical Conductivity Basic Introduction Youtube

Metallic Bond Formation Compounds Expii

Metallic Bonding Gcse Chemistry Combined Science Aqa Revision Study Rocket

Chemical Bonding Metallic Bonding Overview Bonding Ioniccovalentmetallic Structuregiant Ionic Simple Molecular Giant Covalent Giant Metallic Example Ppt Download
(176).jpg)
Chemical Bonding Ionic Covalent Metallic Quiz Proprofs Quiz

Metallic Bonding The Electrostatic Attraction Between Positive Metal Ions And Delocalised Electrons Ppt Download