What Is The Meaning Of Bond Dipole
09042020 A bond dipole is the presence of two opposite charged ends in the same chemical bond. The resultant dipole moment of the entire molecule is the vector sum of all the individual bond dipoles present in that particular molecule.

Dipole Dipole London Dispersion And Hydrogen Bonding Interactions Chemistry Steps
The dipole moment of a molecule is the vector sum of the dipoles of the individual bonds.

What is the meaning of bond dipole. Therefore for measuring dipole moments of molecular bonds a unit called Debye D is used 1D33356410 30 Cm. 02052016 A dipole moment is simply the measure of net polarity in a molecule. The arrow shows the direction of electron flow by pointing toward the more electronegative atom.
D where μ is the bond dipole moment Q is the magnitude of the partial. The partial charges assigned to bonded atoms due to differences in electron density caused by electronegativity inductive effects and other factors. You could have just searched through Google but Im going to answer it anyway.
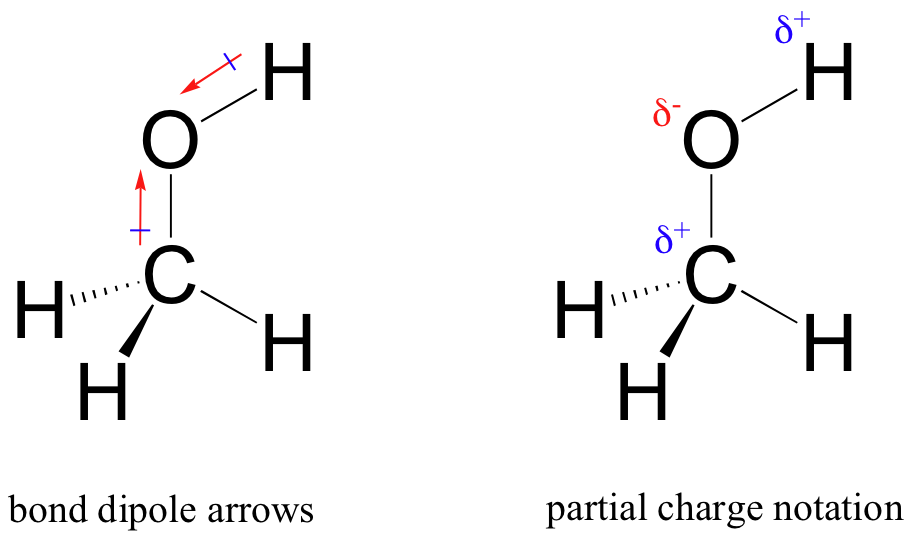
19122017 Dec 20 2017. In HCl for example the dipole moment is indicated as follows. 12082020 Describe the significance of dipole moments Dipole moments are a measure of how much how much charge separation exists in a bond or a molecule.
They occur when there is a separation of charge within a molecule meaning that electrons are shared unequally. A dipole is a type of intermolecular bond between two ions in an ionic bond or two atoms in a covalent bond. 09022021 Simply speaking the dipole is the arrangement of the two charges with different polarity and the dipole moment is the measurement of the electric polarity of the arrangement of these two charges.
02092001 The dipole moment of the bar magnet points from its magnetic south to its magnetic north pole. The charge on the atoms of many substances in the gas phase can be calculated using measured dipole moments and bond. This bond dipole moment is different from the resultant dipole moment of the entire molecule.
However molecular dipole moments are too small to be effectively measured by this unit. Coulomb-meter is an SI unit that applies for the electric dipole moment. This is exactly what occurs in hydrogen bonding.
Dipole dipole interactions occur between the partially negatively charged region the negative dipole and the partially positively charged region the positive dipole of two molecules. Dipole moment definition can be given as the product of magnitude of electronic charge of the molecule and the internuclear distance between the atoms in a molecule. This occurs due to an atoms electronegativity - where one atom has the ability to attract electrons towards it In other words electrons wants to spend other time around it giving it a negative charge and the other a positive charge.
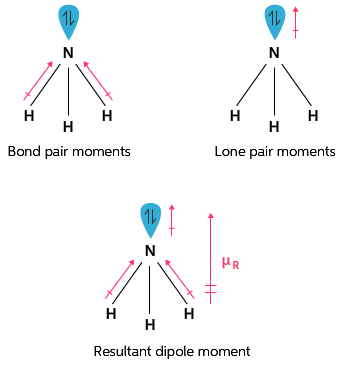
18042019 The important significane of dipole moment are as follows. We can denote bond dipole as. We defined dipoles here when introducing magnetism and discussed polarity also.
01012020 The dipole moment of such a system would be 10 9 coulomb-meter. It is given by the equation. Consider covalently bonded HCl molecule the two atoms share 1 electron each so the shared pair of electrons should be present in between the two atoms but due to that f.
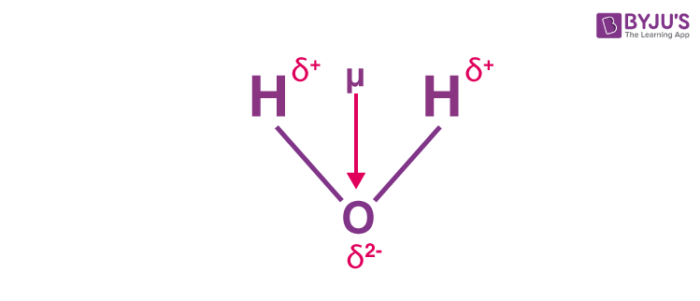
Distance of separation d Q. In a magnetic compass the north pole of a bar magnet points north. This charge separation occurs due to the polarity of the chemical bond.
Dipole moment Charge Q. If a molecule contains polar bonds that are unevenly distributed about the center there will be. However that means that Earths geomagnetic north pole is the south pole south-seeking pole of its dipole moment and vice versa.
A bond dipole moment is an electric dipole moment where there is a positive charge and a negative charge in the same chemical bond. Indicated with δ and δ-andor the arrow symbol. For this reason the strength of a dipole moment depends on differences in electronegativity between the two elements.
Dipole moments occur when there is a separation of charge. We have talked about similar ideas before because molecular dipole moments are important for solvation. For predicting the nature of the molecules- molecules with zero dipole moment are non polar while the molecule with specific dipole moment are polar in nature.
21062021 In a polyatomic molecule the dipole moment of a single bond is known as the bond dipole moment. The opposite charges attract each other forming a temporary bond between the two molecules.

Dipole Moments Mcc Organic Chemistry
Illustrated Glossary Of Organic Chemistry Bond Dipole Bond Dipole Moment
Molecular Polarity Video Vsepr Khan Academy
2 3 Interacciones No Covalentes Libretexts Espanol
Dipole Moment Definition Detailed Explanation And Formula

What Is The General Meaning Of Inducing A Net Dipole Moment On Any Object Quora
Learn About Dipole Moment Of Ammonia Chegg Com