What Is The Meaning Of Electrostatic Bond
The hydrogen bond results from an electrostatic attraction between a positively charged hydrogen ion with negatively charged ions such as O 2 or N 2. Switch to new thesaurus.

Structural Biochemistry Chemical Bonding Ionic Interaction Wikibooks Open Books For An Open World
Relating to or caused by electricity that does not move in a current but is attracted to the.

What is the meaning of electrostatic bond. 16122019 Meanwhile electrostatic interactions describe the attraction force between two completely or partially ionized species with opposite charges. Electrostatic repulsion occurs between two atoms of the same charge. EE Bonds are zero-coupon bonds in that they earn interest monthly but do not pay that interest until they mature or are redeemed.
27032020 Electrostatic attraction is the phenomenon where a negatively charged atom or molecule is attracted to a positively charged atom or molecule. 04112020 EE Bonds are one of two types of savings bond sold by the US. If a failure of electrical insulation occurs all bonded metal objects in the room will have substantially the same electrical potential so that an occupant of the room cannot touch two objects with.
Bond chemical bond - an electrical force linking atoms. Produced or caused by such charges. A bond is a fixed income investment in which an investor loans money to an entity corporate or governmental that borrows the funds for a defined period of time at a fixed interest rate.
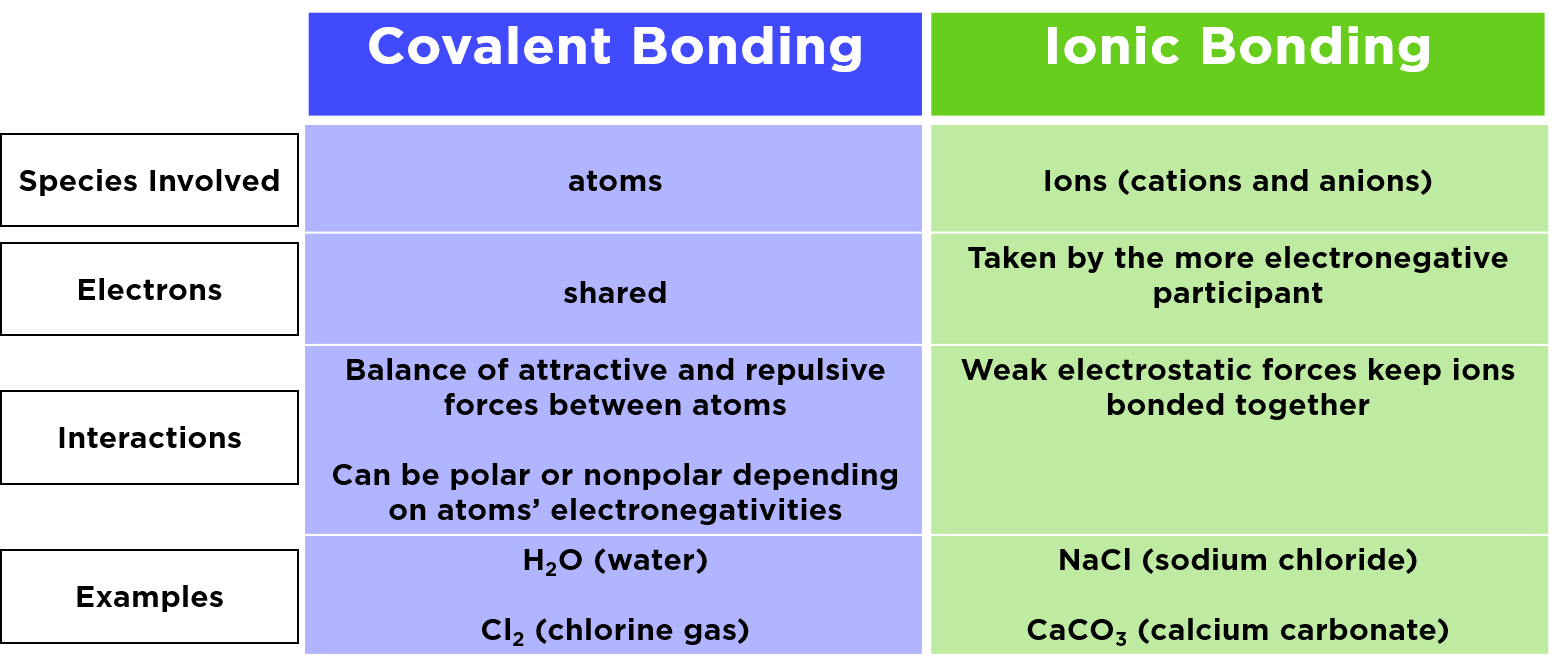
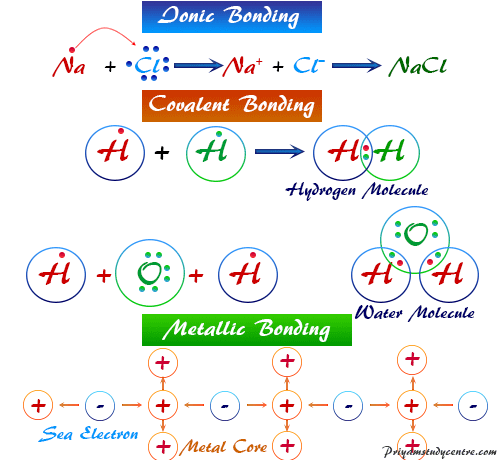
Covalent bond in chemistry the interatomic linkage that results from the sharing of an electron pair between two atoms. The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond. The binding arises from the electrostatic attraction of their nuclei for the same electrons.
The ionic bond of the paint to the metal creates the paint coating in which its thickness is directly proportional to the length of time the parts are left in the tank and the time the charge remains active. Electrostatic bond - a chemical bond in which one atom loses an electron to form a positive ion and the other atom gains an electron to form a negative ion. Analyses of this assessment reveal that emphasizing the significance of electrostatic interactions and the role of potential energy in chemical bonding helps students articulate why atoms form chemical bonds.
This results in hydrogen bonding to its neighbors of opposite charge and holding them together. Ionic and electrostatic interactions are very important chemical concepts that are helpful in determining the buildup of molecules. Electrovalent bond ionic bond.
A bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. These are also named as non-covalent bonds. Treasury the other is I Bonds.
The hydrogen atom may lose its sole electron to any of its neighbors with equal probability. Electrostatic interaction between ionic species of the opposite charges is a relatively strong interaction and often exists in solutions of highly polar solvents especially aqueous solvents. Electrostatic definition is - of or relating to static electricity or electrostatics.
Consequently students recognize the energetics associated with bond formation and dissociation and are able to apply this. An addition to the electrostatic coating or e-coating process is dipping electrically conductive parts into a tank of paint that is then electrostatically charged. Of or relating to electrostatics.
Therefore it can be used in the fluorescent sensing of chiral ions in aqueous environment. Electrostatic synonyms electrostatic pronunciation electrostatic translation English dictionary definition of electrostatic. How Do EE Bonds Work.
A chemical bond is formed between two atoms by the complete transfer of one or more electrons from one atom to the other as a result of which the atoms attain their nearest inert gas configuration. Of or relating to electric charges at rest. Electrical bonding is the practice of intentionally electrically connecting all exposed metal items not designed to carry electricity in a room or building as protection from electric shock.

3 3 Chemical Bonding Biology Libretexts

Review Of Ions Objective When Atoms Give Away Electrons Ionization Energy Electron Configuration Covalent Bonding
Ionic Bonding Biology Definition Role Expii
Chemical Bonding Definition Types Properties Examples

Ib Chemistry Standard Level Notes Covalent Bonding

The Nature Of Bonds A Level Chemistry

What Is N Bromo Succinimide Or Nbs And What Is The Use Of N Bromo Succinimide Or Nbs Chemsolve Net Organic Chemistry Free Radical Chemistry
